
DEN BROK, S.W.J., M. ZAHID, C.W. PASSCHIER (1999): Pressure solution compaction of NaClO3 and implications for pressure solution in NaCl. Tectonophysics 307, 297-312.
Why study pressure solution?
Pressure solution is an important compaction and deformation mechanism in nature, but how it works is not well understood; it cannot yet be successfully modelled, neither in the lab, nor in nature.


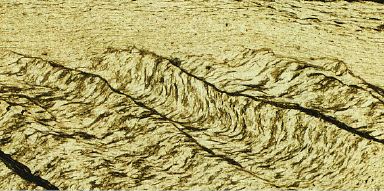
Examples of pressure solution in natural rocks. (a) Dissolution across
quartz grain to grain contact after compaction in sandstone; (b)
Dissolution across stylolites ("fracture cleavage") developed during
progressive crenulation cleavage development in greenschist metamorphic
rocks (from the Apuane Alps, Italy).
Why sodium chlorate?
One way to learn to understand how pressure solution works, is to do experiments with very soluble salts. Mostly, NaCl (halite) has been used for this. The problem with this material, for using it as an analogue for real rocks (like quartz or feldspar), is that it already deforms plastically at very low stresses (of the order of 0.5 MPa) . Sodium chlorate cannot be deformed plastically at room temperature. It fractures at stresses of the order of 15-25 MPa. So, like quartz or feldspar, sodium chlorate is a brittle material at applied stresses and P-T conditions under which pressure solution is studied in the laboratory and in the nature. Like NaCl it is a colourless, optically anisotropic, cubic material, but bonds are covalent, not polar. It has a slightly higher solubility than NaCl (~7 versus ~6 mole/liter water) and a much lower melting temperature: 250-260 degrees Celsius, versus 801 degrees Celsius for NaCl.
A schematic drawing of the compaction apparatus used is presented HERE
and for typical mechanical data: press HERE


Figure 1. (a) and (b) Slight indentation formed by pressure solution during the initial stage of compaction of sodium chlorate aggregates. Grain size approximately 200 Ám. Black specks are mainly polishing powder. Blue colour is the resin in the pores.
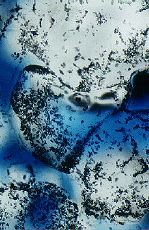

Figure 2. (a) and (b) More intense indentation by pressure solution during the later stages of compaction of sodium chlorate aggregates. Grain size approximately 200 Ám. Black specks are mainly polishing powder. Blue colour is the resin in the pores.

Figure 3. Indentation formed by pressure solution during the final stages of compaction of sodium chlorate aggregates. Grain size approximately 100 Ám. Black specks are mainly polishing powder. Blue colour is the resin in the pores.


Figure 4. (a) and (b) Formation of 120 degrees "equilibrium" grain boundary triple junctions during the final stages of compaction of sodium chlorate aggregates. Grain size approximately 200 Ám. Black specks are mainly polishing powder. Blue colour is the resin in the pores.
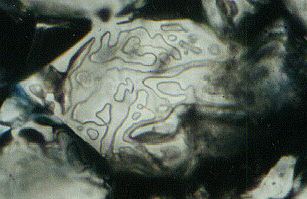
Figure 5. Typical structure of a grain boundary after experiment, showing irregularly distributed tubular fluid inclusions. Grain size approximately 50 Ám.
Goto:
Uniaxial deformation of wet Sodium chlorate aggregates
For comments mail to Moa
Zahid and/or Bas den Brok.
BACK to the Institut für Geowissenschaften in Mainz
BACK to the Geological Institute ETH Zürich